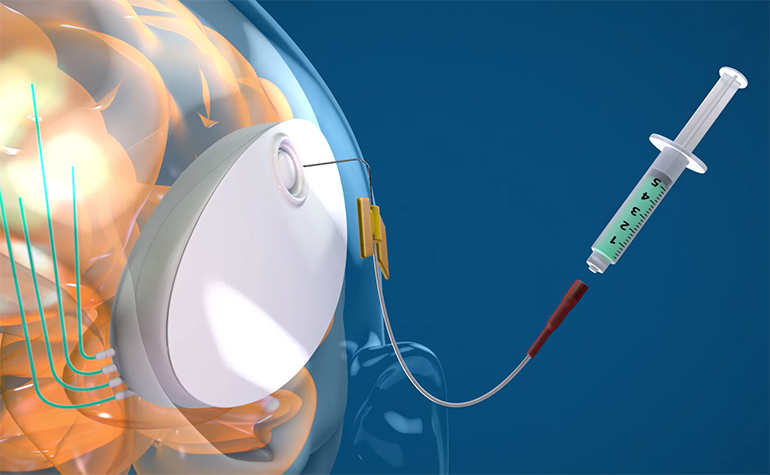
CraniUS, a medtech company based in Baltimore, has developed the NeuroPASS drug delivery system. The technology is designed to deliver drugs to the brain, and it can bypass the blood-brain barrier. This layer of specialized endothelium significantly restricts which drug molecules can enter the brain, normally greatly limiting treatment options for patients with brain-based disease.
The NeuroPASS device is implanted into the skull, where it sits under the scalp. The device inserts catheters into the brain tissue which allow for controlled infusions of drug when required. The implant can be easily refilled from outside, allowing for long-term, minimally invasive drug treatment. It is also wireless and can even be charged wirelessly.
Here’s a quick video introducing the technology:
Medgadget had the opportunity to discuss the technology with Mike Maglin, CEO at CraniUS.
Conn Hastings, Medgadget: Please give us an overview of the blood-brain barrier, and how it affects treatment for a variety of brain-based conditions.
 Mike Maglin, CraniUS: The blood-brain barrier serves as a formidable defense mechanism, protecting the brain from potential harmful substances while allowing essential nutrients to pass through. This selective barrier, composed of specialized endothelial cells, tight junctions, and astrocytes, prevents many therapeutic agents (medicine delivered systemically through pill form or via the bloodstream) from reaching the brain, posing a significant challenge for treating various brain-based conditions. Over 95% of therapeutic drugs’ effectiveness is blocked by the blood brain barrier.
Mike Maglin, CraniUS: The blood-brain barrier serves as a formidable defense mechanism, protecting the brain from potential harmful substances while allowing essential nutrients to pass through. This selective barrier, composed of specialized endothelial cells, tight junctions, and astrocytes, prevents many therapeutic agents (medicine delivered systemically through pill form or via the bloodstream) from reaching the brain, posing a significant challenge for treating various brain-based conditions. Over 95% of therapeutic drugs’ effectiveness is blocked by the blood brain barrier.
Medgadget: What existing techniques are used to bypass this barrier? How are they suboptimal?
Mike Maglin: There are several existing techniques that have been employed to bypass the blood-brain barrier but often face limitations that hinder their efficacy. These limitations include the following:
- In-hospital administration through external catheters that can cause infection over time. In addition, it isn’t sustainable to have patients in-hospital for months at a time seeking this type of treatment.
- Inability to chronically deliver medicine through “one and done” delivery mechanisms
- Inability to easily refill medicine for some implanted devices
- Inability to change flow rates and monitor patient care remotely
- Not MRI safe or compatible. The NeuroPass device resides in important real estate in the skull space with close proximity and access to the brain
Medgadget: What inspired you to develop a drug delivery device that could circumvent the blood-brain barrier?
Mike Maglin: The motivation to develop a drug delivery device capable of circumventing the blood-brain barrier stems from the urgency to revolutionize brain treatment. Witnessing millions of patients struggling with limited treatment options and recognizing the potential of innovative medical technology compelled Dr. Gordon and our team to embark on this endeavor. The NeuroPass device serves as a platform that has the potential to be drug agnostic and treat several chronic brain diseases.
Medgadget: Please give us an overview of the NeuroPASS device, how it is used and what conditions it is suitable for.
Mike Maglin: The NeuroPASS device is a groundbreaking solution designed to navigate the challenges posed by the blood-brain barrier. It is the first fully implantable, wireless medical device that enables chronic and direct delivery of medicine to the brain. It is easily refillable, rechargeable from a distance, and sits invisible under the skin in the skull space.
It is a minimally invasive implantable device that utilizes convection-enhanced delivery (CED) to precisely administer therapeutic agents to the brain. NeuroPASS is suitable for a spectrum of brain-based conditions, ranging from neurodegenerative diseases to brain tumors.
Medgadget: How does the device work? What is convection-enhanced delivery?
Mike Maglin: The device operates by inserting catheters into the brain tissue driven by a pump, allowing for the controlled infusion of therapeutic agents. Convection-enhanced delivery leverages pressure gradients to distribute these agents throughout the affected region with unparalleled precision, overcoming the limitations of passive diffusion.
Medgadget: What are the next steps for the technology, and how do you plan to achieve them?
Mike Maglin: CraniUS recently completed a breakthrough pre-clinical study successfully demonstrating convection enhanced delivery in a swine model. Our roadmap for advancing this technology entails rigorous testing, refinement, and collaboration with experts in neurology, neurosurgery, and medical engineering. We aim to optimize the device’s design for enhanced safety, reliability, and patient comfort and are targeting FDA IND submission and approval in late 2024 to conduct a Phase I first-in-human study in 2025.
The NeuroPASS device represents a significant leap forward in addressing the challenges posed by the blood-brain barrier. By harnessing the power of convection-enhanced delivery, we aspire to transform the landscape of brain treatment and offer hope to countless individuals and their families who have long awaited more effective therapeutic options.
CAUTION – The NeuroPASS device is an investigational device limited by Federal (or United States) law to investigational use and is not available for commercial distribution

